Lipid Nanoparticle News and Research Summary (July 29 - August 18, 2025)
- Aug 17, 2025
- 6 min read

News Summary
Max-Planck-Institut für Kohlenforschung: New study reveals stereochemistry of lipid nanoparticles critically influences safety and efficacy in mRNA delivery
Nagoya University: Japanese researchers engineered a new lipid nanoparticle that delivers mRNA to cells five times more effectively using cyclic disulfide modifications
University of Pennsylvania: Study unlocks safer RNA therapies by reducing harmful inflammation caused by lipid nanoparticles using biodegradable lipids and galectin-blocking drugs
US Department of Health and Human Services: HHS winds down mRNA vaccine development under BARDA, canceling $500 million in funding for 22 mRNA vaccine projects
Multiple Research Institutions: Several journal publications on LNP optimization including transition temperature-guided design, microRNA delivery to CNS, and three-component zwitterionic amino lipids
Brain Delivery Research: New lipid nanoparticle (OS4T LNP) achieved 50-fold increase in mRNA translation within brain tissues compared to FDA-approved formulations
Novel Ionizable Lipid Development: FS01, a new ionizable cationic lipid incorporating ortho-butylphenyl-modified hydrophobic tail, demonstrated superior mRNA delivery and safety profile
Albumin-Recruiting LNPs: Development of albumin-recruiting lipid nanoparticle system with high lymphatic drainage and no liver accumulation to improve mRNA vaccine safety
Detailed News Summaries
Max-Planck-Institut für Kohlenforschung Stereochemistry Study

A collaborative team from the Max-Planck-Institut für Kohlenforschung, Hokkaido University, and Osaka University discovered that the stereochemistry of lipid nanoparticles critically influences safety and efficacy in mRNA delivery. The research, published in the Journal of the American Chemical Society, focused on ALC-315, a key component used in the Pfizer/BioNTech COVID-19 vaccine. The study found that the (S,S) stereoisomer form showed similar efficacy but reduced toxicity compared to the standard mixture, with significant implications for future personalized cancer vaccines and mRNA therapeutics.
Release Date: August 1, 2025
Website Link: https://www.kofo.mpg.de/1032895/2025-08-01-alc-315
Authors: Dr. Chandra Kanta De
Related Institute Location: Max-Planck-Institut für Kohlenforschung (Mülheim an der Ruhr, Germany), Hokkaido University (Sapporo, Japan), Osaka University (Osaka, Japan)
Nagoya University Cyclic Disulfide LNP Enhancement

Japanese researchers at Nagoya University created a new lipid nanoparticle that enhances mRNA delivery into cells by five times through the attachment of sulfur-containing cyclic disulfide ring structures to lipid molecules. The research, published in RSC Medicinal Chemistry, demonstrated that this modification helps mRNA escape cellular endosomes where it normally gets degraded. When tested as a cancer vaccine in mice, the system successfully halted tumor growth with approximately 20% of each nanoparticle's lipid content consisting of the new cyclic disulfide lipids.
Release Date: August 6, 2025
Website Link: https://www.drugtargetreview.com/news/180702/new-lipid-nanoparticle-boosts-mrna-delivery-fivefold-in-cancer-study/
Authors: Dr. Seigo Kimura
Related Institute Location: Integrated Research Consortium on Chemical Sciences, Nagoya University (Nagoya, Japan)
University of Pennsylvania Safer RNA Therapy Research
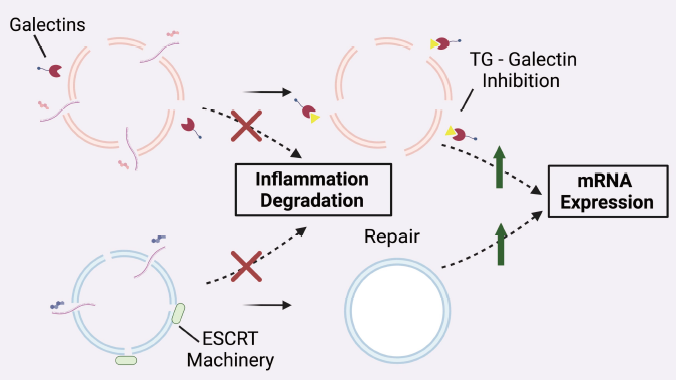
Penn Medicine researchers developed two solutions to reduce harmful inflammation caused by lipid nanoparticles during RNA delivery. The study, published in Nature Nanotechnology, found that adding a unique biodegradable lipid (4A3-SC8) creates smaller endosomal holes that cells can quickly repair, while a drug called thiodigalactoside (TG) blocks inflammation when added to LNPs. These strategies proved transformative in treating acute respiratory distress syndrome (ARDS) in mouse models, dramatically reducing lung inflammation without harmful side effects.
Release Date: August 11, 2025
Website Link: https://www.pennmedicine.org/news/study-unlocks-safer-rna-therapies-for-inflammatory-diseases
Authors: Dr. Jacob Brenner
Related Institute Location: University of Pennsylvania (Philadelphia, PA, USA)
HHS Winds Down mRNA Vaccine Development
The U.S. Department of Health and Human Services announced the termination of mRNA vaccine development activities under BARDA, affecting 22 projects worth approximately $500 million. HHS Secretary Robert F. Kennedy Jr. stated that the decision was based on findings suggesting these vaccines "fail to provide adequate protection against upper respiratory infections such as Covid-19 and influenza." The cancellation includes terminating contracts with Moderna for H5N1 vaccine development, Emory University, Tiba Biotech, and rejecting pre-award solicitations from Pfizer, Sanofi Pasteur, CSL Seqirus, and Gritstone.
Release Date: August 5, 2025
Related Institute Location: U.S. Department of Health and Human Services (Washington, DC, USA)
Standardized LNP Synthesis Protocol
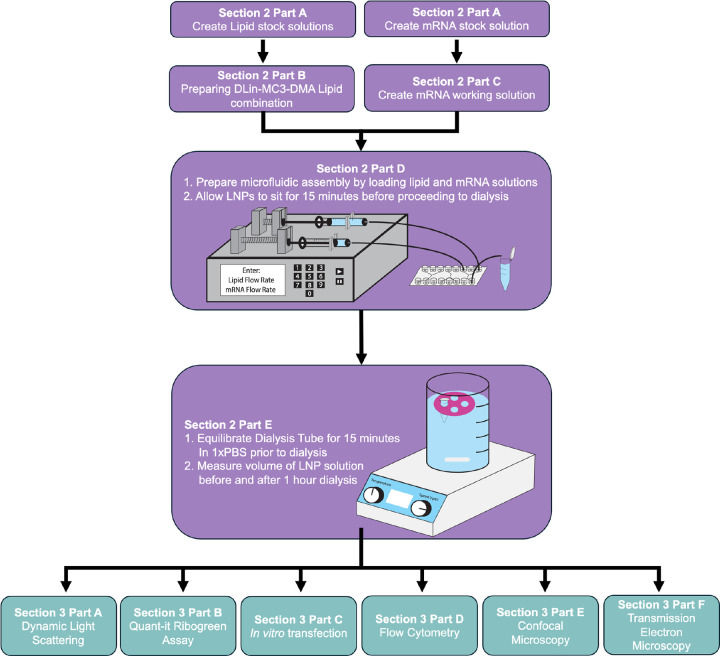
Researchers developed a standardized protocol for microfluidic mixing using cost-effective equipment to synthesize lipid nanoparticles. The protocol, published in a peer-reviewed journal, details synthesis methods using syringe pumps and commercially available microfluidic chips, achieving high encapsulation efficiency (96-100%) across various ionizable lipids including DLin-MC3-DMA, LP01, C12-200, and SM-102. The research demonstrated user-to-user reproducibility even with undergraduate students without prior LNP experience.
Release Date: August 1, 2025
Authors: Shilpi Agrawal, Abbey L Stokes, Christopher E Nelson
Related institute location: Department of Biomedical Engineering, University of Arkansas, Fayetteville, AR, USA and Department of Cell and Molecular Biology, University of Arkansas, Fayetteville, AR, USA.
Overview of methods detailing the order of steps for LNP synthesis and characterization, along with the corresponding sections.
Website Link: https://pubmed.ncbi.nlm.nih.gov/40766677/
Brain-Targeting LNP Development
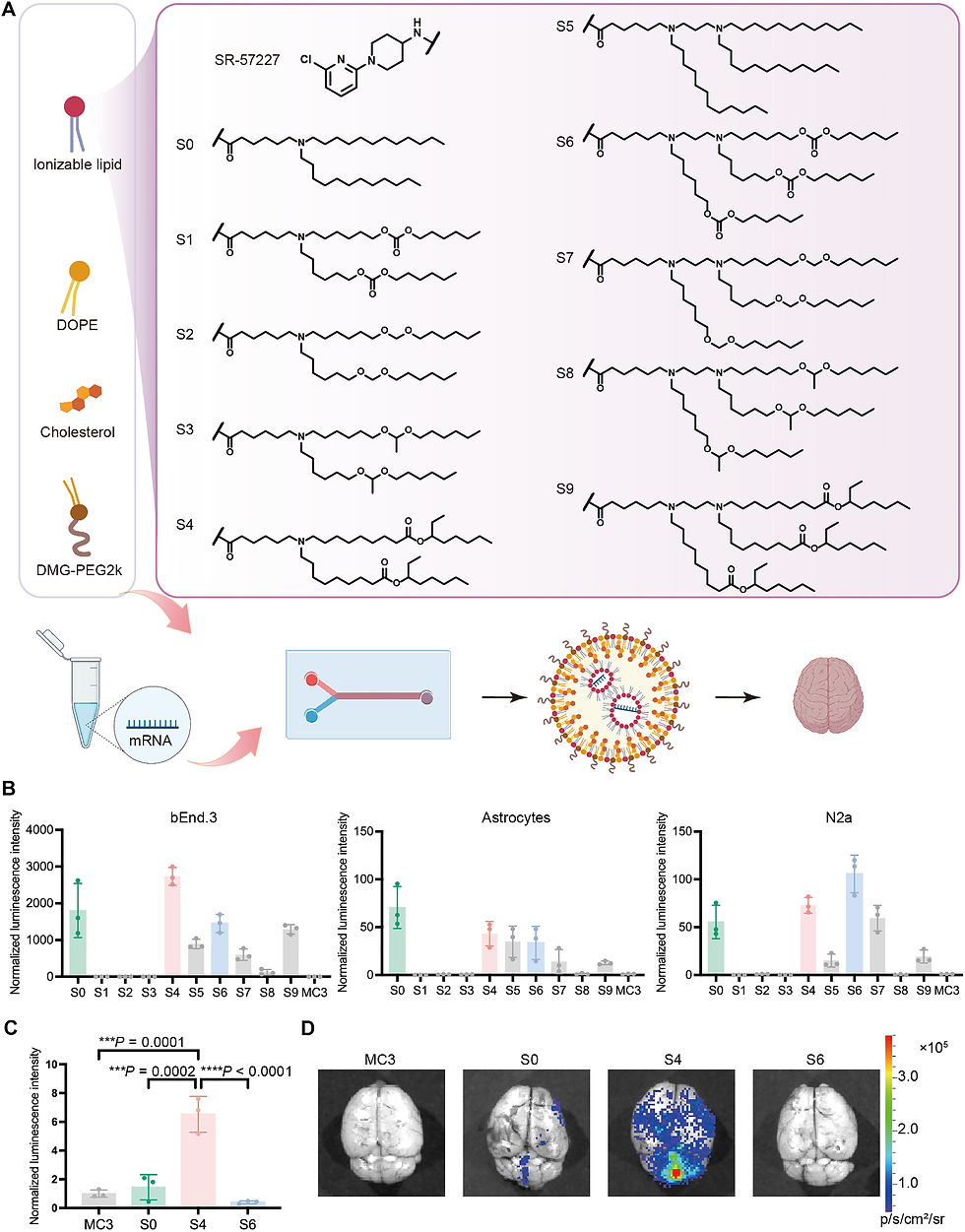
Scientists developed OS4T lipid nanoparticles for mRNA delivery to the brain via systemic administration. Following intravenous administration, OS4T LNPs achieved over a 50-fold increase in mRNA translation within brain tissues compared to FDA-approved formulations, successfully overcoming the blood-brain barrier challenge that has limited mRNA delivery to neural tissues.
Release Date: August 1, 2025
Authors: Chang Wang, Siyu Wang, Zhengwei Liu, Meng Tian, et al.
Website Link: https://www.science.org/doi/10.1126/sciadv.adw0730
Related institution Location: Icahn School of Medicine at Mount Sinai: 135897
MicroRNA-124 LNP for Neuroinflammation
Researchers developed lipid nanoparticle-mediated delivery of microRNA-124 to reduce neuroinflammation. The study, published in Biomaterials, demonstrated that LNPs formulated with S-Ac7-DOG ionizable lipid effectively delivered miR-124 to promote microglial polarization towards an anti-inflammatory phenotype. Both local prefrontal cortex delivery and intravenous injection in LPS-treated mice effectively reduced inflammation markers.
Release Date: July 29, 2025
Authors: Zhanjun Ma, Hong Anh Dang, Jingjing Yang, Giulia Rodella, Ariane Mwema, Emily De Lombaerde, et al.
Website Link: https://pubmed.ncbi.nlm.nih.gov/40753784/Related
Institute Location: Louvain Drug Research Institute, Advanced Drug Delivery and Biomaterials, Université Catholique de Louvain, UCLouvain, 1200, Brussels, Belgium
Three-Component Zwitterionic Amino Lipids
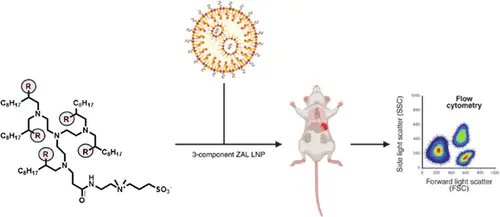
Research published in ACS Biomaterials Science & Engineering explored simplifying lipid nanoparticles from the canonical 4-lipid system to 3-component formulations using Zwitterionic Amino Lipids (ZALs). The study evaluated ZALs modified with opivalate, chloride, bromide, and acetate at the secondary hydroxyl position, with the acetate-modified ZAL showing enhanced immune cell transfection and organ-specific delivery capabilities.
Release Date: July 9, 2025
Author: Joshua J. Robinson, Di Zhang, Pratima Basak, Amogh Vaidya, et al.
Website Link: https://pubs.acs.org/doi/10.1021/acsbiomaterials.5c00631
Related Institute Location: Department of Chemistry and Biochemistry, The University of Texas at Dallas, Richardson, Texas, USA 75080, USA
FS01 Novel Ionizable Lipid Development
Researchers reported the rational design of FS01, a novel ionizable cationic lipid incorporating an ortho-butylphenyl-modified hydrophobic tail into a squaramide-based headgroup architecture. Molecular dynamics simulations revealed that FS01 enhances mRNA stability through π-π stacking interactions and hydrogen bonding. FS01-LNPs demonstrated superior performance across multiple administration routes and showed enhanced delivery efficiency, immunogenicity, and safety compared to FDA-approved lipids.
Release Date: August 14, 2025
Authors: Kun Guo, Lingling Bu, Jintong Du, et al.
Related Institution Location: Innovation Center for Nucleic Acid Medicine, Wuxi Research Center for Innovative Medicines and Life Health, China Pharmaceutical University, Wuxi 214000, China
Website Link: https://pubmed.ncbi.nlm.nih.gov/40818720/
Albumin-Recruiting LNP System
Scientists developed an albumin-recruiting lipid nanoparticle system using Evans blue-modified lipids (EB-LNP) that achieves high lymphatic drainage while avoiding liver accumulation. The system demonstrates albumin-facilitated transport through intramuscular lymphatic vessels to lymph nodes, high dendritic cell internalization, and low blood vessel penetration, resulting in strong cellular and humoral immune responses with minimal toxicity.
Release Date: August 1, 2025
Authors: Yunxuan Feng, Wanbo Tai, Pei Huang, et al.
Related Institution Location: Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology, Department of Chemistry, Tsinghua University, Beijing, China.
Website Link: https://pubmed.ncbi.nlm.nih.gov/40750835/



Comments