Application Note
纳米发电机™纳米粒子合成系统
PreciGenome NanoGenerator™ 是用于纳米粒子合成的高性能仪器,如脂质纳米粒子、脂质体、PLGA 等,广泛用于药物输送、基因治疗、LNP 配制和制造等。
NanoGenerator™ 生成的纳米粒子具有更好的尺寸均匀性和更小的 PDI。它可从 0.1mL/样品筛选扩展到 1L(>10L 定制设计)大批量 GMP 生产
纳米粒子,尤其是脂质体和聚合物纳米粒子,由于其优异的性能,在药物递送、mRNA疫苗和生物传感等制药工业等各个领域显示出巨大的生物医学应用潜力。
通过微流控技术合成纳米颗粒比传统的批量合成工艺具有优势,因为它能够在尺寸和形状上具有更好的均匀性。例如,在药物递送领域,使用NanoGenerator™纳米粒子合成系统可以合成脂质纳米粒子(LNP)、脂质体、PLGA等多种纳米粒子。脂质纳米颗粒 (LNP)、脂质体和 PLGA 是最常用的可生物降解材料,用于输送亲水性和疏水性化合物。
Table 1. Liposome/LNP Tumor Therapy Clinical Trials
Therapy Name | Status/Phase | Cancer Type | Mechanism/Description | Approved/Started Year | Company |
|---|---|---|---|---|---|
NCI-4650 | Phase 1 | Multiple solid tumors | Personalized neoantigen vaccine targeting up to 20 tumor-associated antigens | 2022 (trial start) | National Cancer Institu |
BNT111 | Phase 1 | Melanoma | mRNA vaccine targeting multiple melanoma antigens | 2016 (trial start) | BioNTech |
SYS6020 | Phase 1 | Cell therapy | First mRNA-LNP-based cell therapy approved for clinical trials | 2023 (trial start) | Suzhou YS Biopharma |
CPTX2309 | Phase 1 | Autoimmune (CAR-T) | First in vivo CAR-T therapy; anti-CD19 CAR mRNA via LNP | 2023 (trial start) | Capstan Therapeutics |
SAR441000 | Phase 1 | Solid tumors | mRNA encoding IL-12sc, IL-15sushi, IFNα-2b, GM-CSF | 2019 (trial start) | Sanofi |
mRNA-2752 | Phase 1 | TNBC, melanoma, solid tumors | Intratumoral mRNA encoding OX40L, IL-23, IL-36γ | 2018 (trial start) | Moderna |
BNT116 | Phase 2 | Advanced NSCLC | mRNA vaccine with cemiplimab | 2022 (trial start) | BioNTech |
BNT115 | Phase 2 | Ovarian cancer | mRNA vaccine with neo-adjuvant chemotherapy | 2021 (trial start) | BioNTech |
Autogene Cevumeran (BNT122) | Phase 2 | Pancreatic cancer | Personalized mRNA neoantigen vaccine | 2019 (trial start) | BioNTech / Genentech |
ThermoDox | Phase 3 | Hepatocellular carcinoma | Heat-activated liposomal doxorubicin; triggered release with heating | 2012 (trial start) | Celsion (now Imunon) |
BNT113 | Phase 2/3 | HPV16+ head & neck squamous cell carcinoma | mRNA vaccine with pembrolizumab | 2020 (trial start) | BioNTech |
mRNA-4157 (V940) | Phase 3 | Melanoma, NSCLC, colorectal | Personalized mRNA neoantigen vaccine; breakthrough therapy | 2017 (trial start) | Moderna / Merck |
Ameluz | Approved (EU/US) | Basal cell carcinoma | Liposomal 5-aminolevulinic acid; photodynamic therapy | 2017 | Biofrontera |
Vyxeos | FDA Approved | Acute myeloid leukemia | Liposomal cytarabine + daunorubicin (5:1 ratio); improved survival outcomes | 2017 | Jazz Pharmaceuticals |
Onivyde | FDA Approved | Pancreatic adenocarcinoma | Liposomal irinotecan; improved survival in combination regimens | 2015 | Ipsen (ex. Merrimack, Baxalta) |
Marqibo | FDA Approved | Acute lymphoblastic leukemia | Liposomal vincristine; enables higher dosing, reduced neurotoxicity | 2012 | Spectrum Pharmaceuticals |
Mepact | Approved (EU) | Osteosarcoma | Liposomal mifamurtide; immunomodulator for pediatric osteosarcoma | 2009 | Takeda |
Myocet | Approved (Canada/EU) | Breast cancer | Non-PEGylated liposomal doxorubicin; reduced cardiotoxicity | 2000 | Teva Pharmaceuticals |
DaunoXome | Approved (EU/US) | Kaposi's sarcoma | Liposomal daunorubicin; improved delivery, reduced toxicity | 1996 | Gilead Sciences |
Doxil (Caelyx) | FDA Approved | Ovarian, breast, multiple myeloma, Kaposi's sarcoma | PEGylated liposomal doxorubicin; reduced cardiotoxicity | 1995 | Johnson & Johnson (Janssen), Schering-Plough (legacy) |
1. Personalized Neoantigen and Shared Antigen Immunotherapy
1.1 Personalized Neoantigen Medicine
The most promising application of LNPs in cancer therapy involves personalized neoantigen vaccines that target tumor-specific mutations unique to individual patients. These vaccines represent a paradigm shift toward precision medicine, enabling the development of patient-specific treatments based on comprehensive tumor genomics[5][6]. The process begins with tumor sequencing to identify unique mutations and neoantigens. These are then encoded into mRNA molecules and delivered via LNPs to stimulate robust immune responses.

Fig 1. Neoantigen identification and their derived sources. (A) The neoantigen prediction process follows a systematic three-step pipeline. (B) Assorted mechanisms for neoantigen origin. Image adapted from Journal of Hematology & Oncology. 2025 Feb 17;18(1):18.

Fig 2. Immune responses induced by cancer vaccines. Image adapted from Journal of Hematology & Oncology. 2025 Feb 17;18(1):18.
The mRNA-4157 vaccine, developed by Moderna in collaboration with Merck, exemplifies this approach's clinical potential. In high-risk melanoma patients, this personalized vaccine combined with pembrolizumab demonstrated superior recurrence-free survival, with 18-month recurrence-free survival rates of 78.6% compared to 62.2% for pembrolizumab monotherapy[6][7]. This success led to FDA Breakthrough Therapy Designation in February 2023, highlighting the transformative potential of LNP-delivered personalized cancer vaccines.
Clinical trials have demonstrated the feasibility of targeting up to 34 personalized neoantigens simultaneously, significantly expanding the breadth of immune responses while reducing the risk of immune escape[6][7]. The BNT111 vaccine targets multiple melanoma-associated antigens including NY-ESO-1, tyrosinase, MAGE-A3, and TPTE, showing promising results in combination with anti-PD-1 therapy, with 35% of patients experiencing partial responses.
1.2 Shared Antigen Vaccines
In addition to personalized approaches, LNPs deliver vaccines targeting shared tumor-associated antigens across multiple patients. The CV9201 and CV9202 vaccines target antigens including MAGE-C1, MAGE-C2, NY-ESO-1, survivin, and 5T4 in non-small cell lung cancer, demonstrating the versatility of LNP platforms across different cancer types[7]. While these vaccines showed limited objective responses as monotherapy, they provided valuable insights into antigen selection and combination strategies.
2. Immunotherapy Applications
2.1 Cytokine Delivery Systems
LNPs have revolutionized cytokine delivery for cancer immunotherapy by enabling localized expression of potent immune-stimulating molecules while minimizing systemic toxicity. Intratumoral delivery of LNPs encoding cytokines like IL-12, IL-21, and IL-7 has shown remarkable anti-tumor activity in preclinical studies[8][9]. The combination of IL-21, IL-7, and 4-1BB ligand delivered via LNPs achieved complete tumor regression in 6 out of 7 MC38 tumor models, significantly outperforming individual treatments.
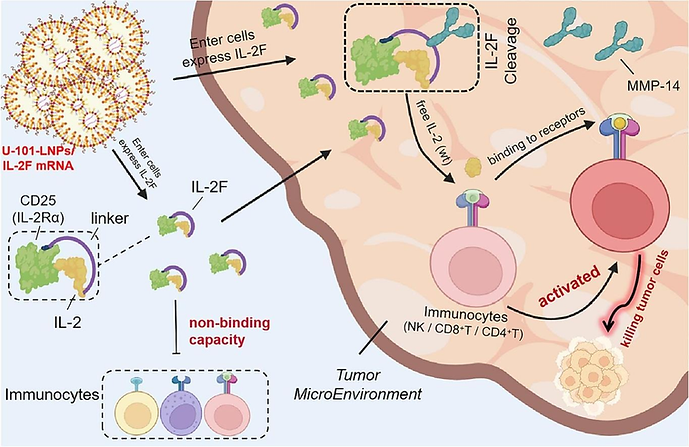
Fig 3. U-101-LNP/IL-2F mRNA expresses IL-2F in vivo. Image adapted from Journal of Controlled Release. 2024 Apr 1;368:663-75.
Previously, clinical trials by Moderna have evaluated mRNA-2416, an LNP formulation encoding human OX40L, for intratumoral delivery in solid tumors and lymphomas. While the trial was terminated early due to insufficient efficacy endpoints, it demonstrated the safety and tolerability of LNP-based cytokine delivery and provided valuable insights for future development[7].
2.2 Immune Checkpoint Modulation
LNPs enable sophisticated approaches to immune checkpoint modulation beyond traditional antibody-based therapies. The delivery of mRNA encoding checkpoint inhibitors or modulators directly to tumor sites offers the potential for enhanced local efficacy while reducing systemic side effects. Additionally, LNP-based vaccines can resensitize tumors to checkpoint inhibitor therapy, particularly in models previously resistant to anti-PD-1 treatment[8][10].
3. Gene Therapy Applications
3.1 CRISPR-Cas9 Delivery
LNPs have emerged as superior delivery vehicles for CRISPR-Cas9 gene editing systems in cancer therapy. These formulations enable efficient delivery of guide RNAs, Cas9 mRNA, and donor templates to tumor cells for precise genetic modifications[11][12]. Recent studies demonstrated LNP-mediated delivery of CRISPR components targeting the PLK1 gene in liver cancer, achieving significant tumor growth suppression without adverse effects on major organs[13].

Fig 4. Schematic illustration for targeting the mechanical properties of tumors to open a double-checkpoint blockade of cancer (stiff ECM plus immunosuppression) to enable cancer therapy. Proposed model for how dendrimer LNPs encapsulating FAK siRNA, Cas9 mRNA and targeted sgRNAs could exhibit enhanced penetration into tumours with increased gene editing of PD-L1 for improved cancer therapy. Image adapted from Nature nanotechnology. 2022 Jul;17(7):
The development of targeted LNPs for CRISPR delivery has shown particular promise in ovarian cancer models, where specialized formulations achieved up to 80% gene editing efficiency, resulting in improved survival and reduced tumor burden[11]. These advances represent a significant step toward precise, programmable cancer therapy through targeted gene editing.
3.2 siRNA Therapeutics
Small interfering RNA (siRNA) delivery via LNPs offers targeted approaches to silencing oncogenes and drug resistance genes. The clinical development of TKM-080301, an LNP-formulated siRNA targeting PLK1, demonstrated the feasibility of this approach in solid tumors, including gastrointestinal neuroendocrine tumors and adrenocortical carcinoma[14]. While the trial showed limited anti-tumor effects as monotherapy, it provided important safety data and proof-of-concept for siRNA-based cancer therapy.

Fig 5. Schematic of brain tumor immunotherapy mediated by BAMPA-O16B/siRNA lipoplex. Image adapted from Biomaterials. 2022 Aug 1;287:121645.
Co-delivery of siRNA and chemotherapeutic agents using LNPs has shown synergistic effects in overcoming drug resistance. The combination of cisplatin with siRNA targeting DNA repair genes demonstrated enhanced cytotoxicity in drug-resistant lung cancer cells, representing a significant advancement in combination therapy approaches[15].
4. Targeted Drug Delivery
4.1 Enhanced Permeability and Retention (EPR) Effect
LNPs leverage the EPR effect for passive tumor targeting, taking advantage of the abnormal vasculature and impaired lymphatic drainage characteristic of solid tumors.
Nanoparticles in the optimal size range of 50-200 nm can extravasate through fenestrated tumor vasculature and accumulate in tumor tissues, achieving preferential localization while minimizing exposure to healthy tissues[16].
This passive targeting mechanism has proven particularly effective for delivering chemotherapeutic agents with improved therapeutic indices. Formulations like liposomal doxorubicin (Doxil) have transformed cancer treatment by maintaining anti-tumor efficacy while significantly reducing cardiotoxicity compared to free drug administration.
4.2 Active Targeting Strategies
Advanced LNP formulations incorporate active targeting mechanisms using ligands such as antibodies, peptides, or small molecules to direct nanoparticles to specific cell surface receptors overexpressed on tumor cells[17][18]. Recent developments include PD-L1-targeted LNPs that deliver PTEN mRNA specifically to triple-negative breast cancers, demonstrating enhanced uptake and therapeutic efficacy compared to non-targeted formulations[19][17].

Fig 6. Representative surface modification strategies for LNP targeting. Image adapted from Advanced Materials. 2023 Dec;35(51):2303261.
The integration of tumor-targeting peptides through click chemistry has enabled the development of sophisticated targeting systems that combine passive and active mechanisms for enhanced tumor specificity[17][18]. These approaches represent a significant advancement in precision drug delivery for cancer therapy.
5. Manufacturing and Scalability
5.1 Production Technologies
The manufacturing of LNPs for cancer therapeutics requires sophisticated production technologies that ensure consistent quality, scalability, and cost-effectiveness. Microfluidic mixing technologies have emerged as the gold standard for LNP production, enabling precise control over particle size, composition, and drug loading[20][21]. These systems can achieve production rates of several L/h while maintaining consistent product quality[20].

Fig 7. LNP synthesis with microfluidic mixing
Recent developments in scalable microfluidic platforms have addressed manufacturing challenges through the integration of hundreds of mixing units on single chips, enabling production rates suitable for clinical applications[20][21]. The SCALAR platform, fabricated entirely from silicon and glass substrates, demonstrates the feasibility of directly translating microfluidic-generated LNPs to commercial production scales[20].

Fig 8. Scaling up LNP drug development
5.2 Quality Control and Standardization
The complex nature of LNP formulations requires comprehensive characterization and quality control measures to ensure reproducibility and safety. Critical quality attributes include particle size distribution, encapsulation efficiency, stability, and biological activity[22]. The development of standardized characterization protocols has improved the reproducibility and comparability of LNP products across different manufacturers and research institutions.
6. Safety Profile and Toxicity Considerations
6.1 Biocompatibility and Immunogenicity
The safety profile of LNPs is generally favorable, with most formulations demonstrating good biocompatibility and limited toxicity at therapeutic doses[13]. However, several safety considerations require careful evaluation, including the potential for ionizable lipids to activate inflammatory pathways and the immunogenicity of repeated administrations.
Recent research has focused on developing LNPs with reduced immunogenicity through the use of biodegradable components and optimized lipid compositions[13]. The replacement of PEGylated lipids with alternative stealth components such as polysarcosine has shown promise in reducing complement activation and hypersensitivity reactions.
6.2 Dose-Dependent Effects and Long-Term Safety
The safety profile of LNPs is highly dose-dependent, with toxicity typically observed only at doses exceeding therapeutic requirements[13]. Local reactions at injection sites are common with intratumoral administration but are generally mild and transient. Long-term safety studies have demonstrated the absence of chronic toxicity with repeated LNP administration, supporting their use in chronic treatment regimens.
7. Challenges and Future Directions
7.1 Technical Challenges
Despite significant progress, several technical challenges remain in optimizing LNP-based cancer therapeutics. The enhancement of targeting specificity continues to be a priority, as current systems often achieve only modest improvements in tumor selectivity[13][23]. The development of more sophisticated targeting mechanisms, including cell-specific ligands and tissue-specific promoters, represents an active area of research.
The stability of LNP formulations under physiological conditions presents ongoing challenges, particularly for long-term storage and global distribution[23]. While advances in formulation science have addressed many stability issues, further improvements are needed for widespread clinical deployment.
7.2 Manufacturing and Cost Considerations
Manufacturing scalability and cost-effectiveness represent significant barriers to widespread clinical adoption of LNP-based cancer therapeutics[24]. The complex manufacturing requirements and personalized nature of many treatments may limit accessibility, particularly in resource-limited settings. The development of automated manufacturing platforms and point-of-care production technologies could address these challenges[24].
7.3 Emerging Opportunities
The integration of artificial intelligence and machine learning approaches is accelerating the discovery and optimization of new LNP formulations for cancer therapy[24][25]. These technologies enable rapid screening of lipid compositions, prediction of targeting efficiency, and optimization of manufacturing processes.
The development of next-generation LNP platforms focuses on tissue-specific targeting systems, improved stability formulations, and enhanced manufacturing processes[25]. Stimuli-responsive LNPs that release payloads in response to specific tumor microenvironment conditions represent an exciting frontier that could significantly improve therapeutic specificity[26].
8. Conclusion
The application of lipid nanoparticles in tumor therapy represents a transformative advancement in cancer treatment, offering unprecedented opportunities for personalized, targeted, and effective therapy. The remarkable versatility of LNP platforms has enabled successful applications across multiple therapeutic modalities, from personalized mRNA vaccines to sophisticated gene therapy approaches. The clinical success of LNP-based treatments, exemplified by breakthrough therapy designations and advancing Phase III trials, demonstrates the real-world potential of this technology.
Current applications span personalized neoantigen vaccines, immunotherapy enhancement, gene editing, targeted drug delivery, and combination therapies, with over 120 clinical trials evaluating diverse approaches. The regulatory landscape has become increasingly supportive, with streamlined pathways for LNP-based therapeutics building on the success of approved formulations.
While challenges remain in targeting specificity, manufacturing scalability, and cost-effectiveness, these are being actively addressed through innovative research and development efforts. The integration of artificial intelligence, advanced manufacturing processes, and novel therapeutic approaches is expected to further enhance the efficacy and accessibility of LNP-based cancer treatments.
The future of LNP-based cancer therapeutics is exceptionally promising, with ongoing developments in personalized medicine, combination therapies, and precision targeting. As the field continues to mature, LNPs are positioned to play an increasingly central role in the evolution of cancer therapy, offering hope for improved outcomes and quality of life for cancer patients worldwide. The collaborative efforts of academic institutions, biotechnology companies, and regulatory agencies are accelerating the translation of promising research into clinical reality, supporting the expectation that LNP-based platforms will become standard components of cancer treatment in the coming decades.
References:
-
https://www.sciencedirect.com/science/article/abs/pii/S2666634024004562
-
https://www.cas.org/resources/cas-insights/lipid-nanoparticles-key-players-in-cancer-treatment
-
https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2024.1296091/full
-
https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202411032
-
https://innovativegenomics.org/news/crispr-clinical-trials-2024/
-
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.967505/full
-
https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202309917
-
https://www.frontiersin.org/journals/oncology-reviews/articles/10.3389/or.2025.1541326/full


